Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall resul...

Chemistry, 06.10.2019 01:30 hayleylatti8691
Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall result when ch4(g) burns
according to this reaction?
(1) energy is absorbed and dh is negative.
(2) energy is absorbed and dh is positive.
(3) energy is released and dh is negative.
(4) energy is released and dh is positive.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Questions

English, 10.06.2021 06:50


Mathematics, 10.06.2021 06:50







Mathematics, 10.06.2021 06:50



Spanish, 10.06.2021 06:50




Biology, 10.06.2021 06:50

Mathematics, 10.06.2021 06:50


Chemistry, 10.06.2021 06:50

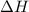 is negative.
is negative.


