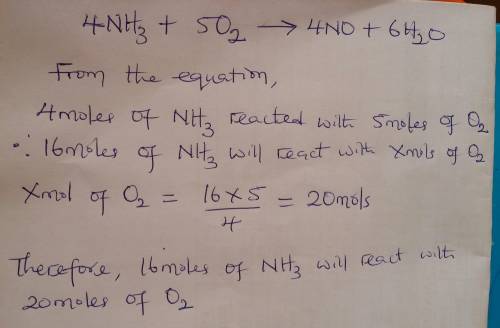Given the balanced equation representing a reaction:
4nh3 + 5o2 ==> 4no + 6h2o
what...

Chemistry, 17.11.2019 07:31 michelle5642b
Given the balanced equation representing a reaction:
4nh3 + 5o2 ==> 4no + 6h2o
what is the minimum number of moles of o2 that are needed to completely react with 16 moles of nh3?
(1) 16 mol (3) 64 mol
(2) 20. mol (4) 80. mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Questions

Mathematics, 09.03.2022 23:40


Mathematics, 09.03.2022 23:40

History, 09.03.2022 23:40


Mathematics, 09.03.2022 23:40

Mathematics, 09.03.2022 23:40



Spanish, 09.03.2022 23:40

Chemistry, 09.03.2022 23:40

History, 09.03.2022 23:40


Mathematics, 09.03.2022 23:40



History, 09.03.2022 23:50

Mathematics, 09.03.2022 23:50