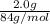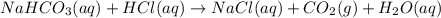If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon dioxide should one tablet yield? compare this theoretical value with your results. (hint: you will first need to convert your mass into moles by dividing by the molar mass of nahco3 or sodium hydrogen carbonate a/k/a baking soda). , show all work and think about mole ratios relating co2 to nahco3.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon di...
Questions





Business, 09.09.2021 07:40

History, 09.09.2021 07:40











Mathematics, 09.09.2021 07:40


Health, 09.09.2021 07:40

Mathematics, 09.09.2021 07:40

 (1)
(1)