
Chemistry, 19.12.2019 03:31 Theacefamily123
Which statement explains why br2 is a liquid at stp and i2 is a solid at stp? (1) molecules of br2 are polar, and molecules of i2 are nonpolar.
(2) molecules of i2 are polar, and molecules of br2 are nonpolar.
(3) molecules of br2 have stronger intermolecular forces than molecules of i2.
(4) molecules of i2 have stronger intermolecular forces than molecules of br2.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Which statement explains why br2 is a liquid at stp and i2 is a solid at stp? (1) molecules of br2 a...
Questions

Mathematics, 08.12.2020 01:40

Geography, 08.12.2020 01:40

English, 08.12.2020 01:40

Arts, 08.12.2020 01:40

Social Studies, 08.12.2020 01:40


Mathematics, 08.12.2020 01:40

Mathematics, 08.12.2020 01:40


Mathematics, 08.12.2020 01:40




Computers and Technology, 08.12.2020 01:40





Mathematics, 08.12.2020 01:40

English, 08.12.2020 01:40

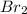 is a liquid at STP and
is a liquid at STP and 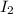 is a solid at STP because molecules of
is a solid at STP because molecules of 

