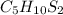Chemistry, 02.08.2019 09:30 merunikitty1226
Analysis of skunk spray yields a molecule with 44.77% c, 7.46% h and 47.76% s by mass. what is the empirical formula for this molecule found in the spray of skunks that scientists think is partly responsible for the strong odor?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Analysis of skunk spray yields a molecule with 44.77% c, 7.46% h and 47.76% s by mass. what is the e...
Questions

Mathematics, 28.10.2020 06:20






English, 28.10.2020 06:20


Mathematics, 28.10.2020 06:20

Computers and Technology, 28.10.2020 06:20

English, 28.10.2020 06:20

Chemistry, 28.10.2020 06:20


English, 28.10.2020 06:20

Mathematics, 28.10.2020 06:20

Mathematics, 28.10.2020 06:20


Arts, 28.10.2020 06:20

Mathematics, 28.10.2020 06:20

Biology, 28.10.2020 06:20