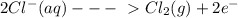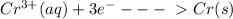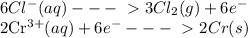Chemistry, 02.08.2019 05:30 orlando19882000
The information below describes a redox reaction. cr3+(aq)+2cl-(> cr(s)+cl2(s) 2cl-(> cl2(g)+2e- cr3+(aq)+3e- > cr(s) what is the final, balanced equation for this reaction? 1.) 2cr3+(aq)+6cl-(aq) > 2cr(s)+3cl2(g) 2.) 2cr3(aq)+2cl-(aq)+6e- > cl2(g)+2cr(s) 3.) cr3+(aq)+6cl-(aq)+3e- > 2cr(g)+3cl2(g) 4.) cr3+(aq)+2cl-(> cr(s)+cl2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
The information below describes a redox reaction. cr3+(aq)+2cl-(> cr(s)+cl2(s) 2cl-(> cl2(g)+2...
Questions







Computers and Technology, 24.02.2020 23:29


Physics, 24.02.2020 23:29