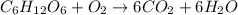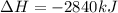Chemistry, 01.08.2019 16:30 carlalopezelox2244
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), according to the equation below. mc020-1.jpg the enthalpy of the reaction is –2,840 kj. what is the heat of combustion, per mole, of glucose? –2,840 kj/mol –473.3 kj/mol 473.3 kj/mol 2,840 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), acco...
Questions

Biology, 24.04.2020 03:27




Mathematics, 24.04.2020 03:27




Mathematics, 24.04.2020 03:27

Mathematics, 24.04.2020 03:27



Mathematics, 24.04.2020 03:27

Mathematics, 24.04.2020 03:27





Mathematics, 24.04.2020 03:27