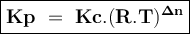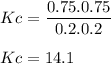Chemistry, 01.08.2019 16:30 afloareiandrei8615
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00 l container. co(g)+h2o(g)↽−−⇀co2(g)+h2(g) how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol once equilibrium has been reestablished?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
An equilibrium mixture contains 0.750 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions

Computers and Technology, 01.08.2019 06:30

Computers and Technology, 01.08.2019 06:30

Computers and Technology, 01.08.2019 06:30




Biology, 01.08.2019 06:30


History, 01.08.2019 06:30

Social Studies, 01.08.2019 06:30

Mathematics, 01.08.2019 06:30


Physics, 01.08.2019 06:30

Chemistry, 01.08.2019 06:30



Computers and Technology, 01.08.2019 06:30

Social Studies, 01.08.2019 06:30

Arts, 01.08.2019 06:30

Mathematics, 01.08.2019 06:30

![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0158/5375/9d1db.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0158/5375/b3cf6.png)