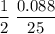Chemistry, 01.08.2019 16:00 cia196785920
Consider the reaction 2 hbr (g) à h2 (g) + br2 (g) a. express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. in the first 25.0 s of this reaction, the concentration of hbr drops from 0.600 m to 0.512 m. calculate the average rate of the reaction during this time interval.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Consider the reaction 2 hbr (g) à h2 (g) + br2 (g) a. express the rate of the reaction in terms of t...
Questions



Mathematics, 06.11.2019 02:31

Health, 06.11.2019 02:31



English, 06.11.2019 02:31

English, 06.11.2019 02:31

Mathematics, 06.11.2019 02:31



Geography, 06.11.2019 02:31

Social Studies, 06.11.2019 02:31

English, 06.11.2019 02:31


History, 06.11.2019 02:31

Mathematics, 06.11.2019 02:31


History, 06.11.2019 02:31

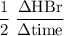 =
= 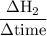 =
= 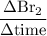 .
. 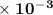 M/s.
M/s.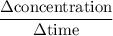
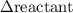 has been the change in reactants concentration, and
has been the change in reactants concentration, and 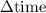 has been the change in the time.
has been the change in the time. = 0.60 M - 0.512 M
= 0.60 M - 0.512 M