Chemistry, 01.08.2019 12:00 mcclendoncassandra
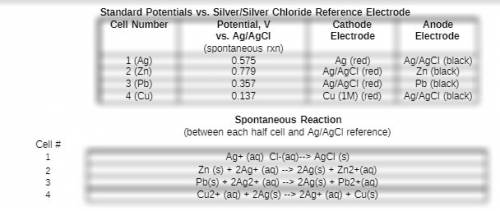
Based on your measured potential for this cell and the literature value for the standard reduction potential for the ag/agcl reference electrode, what would you expect the overall potential to be for the spontaneous reaction between your cu2+/cu electrode and a standard hydrogen eletrode? type your calculation for the expected standard reduction potential vs the she as well as the % error between this value and the literature value.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 23.06.2019 14:00
Which of the following represents the balanced reduction half-reaction from the redox reaction? (2 points) pb + pd(no3)2 yields pb(no3)2 + pd pb yields pd2+ + e- pd2+ + 2e- yields pd pb + e- yields pb 2 pd2+ + 4 e- yields 2 pd
Answers: 1

Chemistry, 23.06.2019 17:30
Iput 40 points on !i need an a or my mom is gonna kill me! 1. what three characteristics does grain perfect fabric have? 2. what do you need to know to identify your pattern layout on the guide sheet? 3. define: pressing 4. define: interfacing 5. define: design seam 6. when should you preshrink your fabric?
Answers: 1
You know the right answer?
Based on your measured potential for this cell and the literature value for the standard reduction p...
Questions



Chemistry, 28.07.2021 20:50

Business, 28.07.2021 20:50

Mathematics, 28.07.2021 20:50



Arts, 28.07.2021 20:50

Health, 28.07.2021 20:50


Physics, 28.07.2021 20:50

English, 28.07.2021 20:50



Advanced Placement (AP), 28.07.2021 20:50




Chemistry, 28.07.2021 20:50