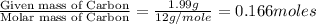Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

You know the right answer?
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 5.00 g of thi...
Questions

Biology, 31.10.2019 08:31


Advanced Placement (AP), 31.10.2019 08:31

Mathematics, 31.10.2019 08:31

Mathematics, 31.10.2019 08:31






Social Studies, 31.10.2019 08:31

Mathematics, 31.10.2019 08:31

History, 31.10.2019 08:31

Mathematics, 31.10.2019 08:31

Social Studies, 31.10.2019 08:31

History, 31.10.2019 08:31

Mathematics, 31.10.2019 08:31


English, 31.10.2019 08:31

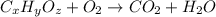
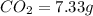
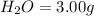
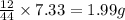 of carbon will be contained.
of carbon will be contained.