
Chemistry, 01.08.2019 04:30 natalie407888
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon? (2 points) a. the argon sample would have the same number of atoms as the neon sample. b. the argon sample would have twice as many atoms as the neon sample. c. the argon sample would have four times as many atoms as the neon sample. d. the argon sample would have eight times as many atoms as the neon sample.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic m...
Questions








Social Studies, 24.01.2020 22:31

History, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31


History, 24.01.2020 22:31


Mathematics, 24.01.2020 22:31






History, 24.01.2020 22:31

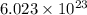 of particles.
of particles.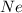 contains=
contains=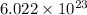 atoms
atoms 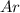 contains=
contains=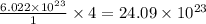 atoms
atoms

