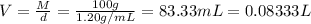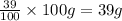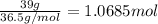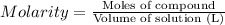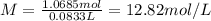Chemistry, 01.08.2019 02:30 mothertrucker2828
Commercial grade hcl solutions are typically 39.0% (by mass) hcl in water. determine the molarity of the hcl, if the solution has a density of 1.20 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Commercial grade hcl solutions are typically 39.0% (by mass) hcl in water. determine the molarity of...
Questions

Mathematics, 21.07.2020 01:01

Health, 21.07.2020 01:01

Mathematics, 21.07.2020 01:01


Mathematics, 21.07.2020 01:01



Mathematics, 21.07.2020 01:01


Mathematics, 21.07.2020 01:01

Mathematics, 21.07.2020 01:01



Mathematics, 21.07.2020 01:01





Biology, 21.07.2020 01:01