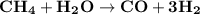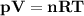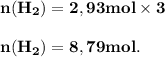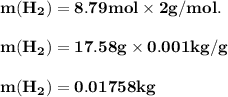Chemistry, 31.07.2019 18:30 katlynnschmolke
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. suppose a chemical engineer studying a new catalyst for the reform reaction finds that 159. liters per second of methane are consumed when the reaction is run at 294.°c and 0.86atm . calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of...
Questions

Social Studies, 14.12.2020 22:00



Mathematics, 14.12.2020 22:00




Mathematics, 14.12.2020 22:00

Health, 14.12.2020 22:00


Business, 14.12.2020 22:00

Geography, 14.12.2020 22:00



Mathematics, 14.12.2020 22:00


Physics, 14.12.2020 22:00


Business, 14.12.2020 22:00