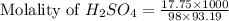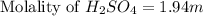Chemistry, 31.07.2019 15:00 dominikkovar
Asolution is prepared by dissolving 17.75 g sulfuric acid, h2so4, in enough water to make 100.0 ml of solution. if the density of the solution is 1.1094 g/ml, what is the molality?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 17.75 g sulfuric acid, h2so4, in enough water to make 100.0 ml o...
Questions


Mathematics, 26.01.2021 01:40


Mathematics, 26.01.2021 01:40

Social Studies, 26.01.2021 01:40

Advanced Placement (AP), 26.01.2021 01:40

Mathematics, 26.01.2021 01:40



Mathematics, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40







Social Studies, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40

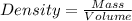
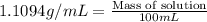
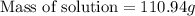
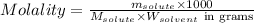
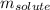 = Given mass of solute
= Given mass of solute 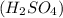 = 17.75 g
= 17.75 g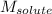 = Molar mass of solute
= Molar mass of solute 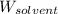 = Mass of solvent = 93.19 g
= Mass of solvent = 93.19 g