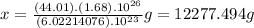Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1
You know the right answer?
How many grams are in 1.68 x 1026 molecules of co2? (molar mass=44.01 g/mol) (hint: you will need...
Questions





Mathematics, 31.01.2021 22:00


Health, 31.01.2021 22:00


English, 31.01.2021 22:00

Mathematics, 31.01.2021 22:00


Chemistry, 31.01.2021 22:00

Biology, 31.01.2021 22:00


English, 31.01.2021 22:00



Social Studies, 31.01.2021 22:00

Mathematics, 31.01.2021 22:00

Mathematics, 31.01.2021 22:00

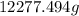 of CO2
of CO2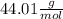
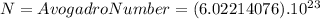
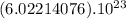 molecules of CO2 (1 mole of CO2) there is 44.01 g of CO2 ⇒
molecules of CO2 (1 mole of CO2) there is 44.01 g of CO2 ⇒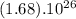 molecules of CO2 there will be x grams :
molecules of CO2 there will be x grams :