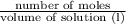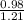Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
If 0.98 moles of bacl2 are dissolved in enough water to make a 1.2-liter solution, what is the resul...
Questions

Mathematics, 19.10.2019 23:00


Mathematics, 19.10.2019 23:00



Mathematics, 19.10.2019 23:00


Mathematics, 19.10.2019 23:00

Mathematics, 19.10.2019 23:00

Mathematics, 19.10.2019 23:00


English, 19.10.2019 23:00


English, 19.10.2019 23:00


Mathematics, 19.10.2019 23:00

Mathematics, 19.10.2019 23:00

History, 19.10.2019 23:00

Mathematics, 19.10.2019 23:00