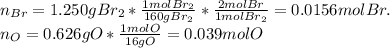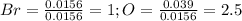Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 08:00
How many distinct monochlorinated products, including stereoisomers, can result when the alkane below is heated in the presence of cl2? 3 4 5 6 7?
Answers: 3

Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1

Chemistry, 23.06.2019 15:00
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3

Chemistry, 23.06.2019 16:10
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2
You know the right answer?
Oxides of virtually every element are known. bromine, for example, forms several oxides when treated...
Questions

Mathematics, 12.12.2020 16:30



Computers and Technology, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Chemistry, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30


English, 12.12.2020 16:30



English, 12.12.2020 16:30

English, 12.12.2020 16:30

Advanced Placement (AP), 12.12.2020 16:30

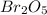

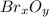 because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the
because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the