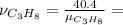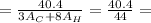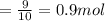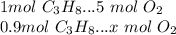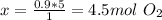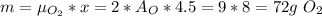The combustion of propane may be described by the chemical equation,
c3h8(g) + 5o2(g) -->...

Chemistry, 21.08.2019 12:00 natachalebrun2
The combustion of propane may be described by the chemical equation,
c3h8(g) + 5o2(g) --> 3co2(g) + 4h2o(g)
how many grams of o2(g) are needed to completely burn 40.4 g of c3h8(g)?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
Questions




Spanish, 14.11.2020 01:00


Mathematics, 14.11.2020 01:00

English, 14.11.2020 01:00

Mathematics, 14.11.2020 01:00




English, 14.11.2020 01:00


History, 14.11.2020 01:00



Mathematics, 14.11.2020 01:00

History, 14.11.2020 01:00

English, 14.11.2020 01:00