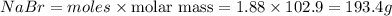Chemistry, 16.12.2019 02:31 devbar3416
In the following reaction, how many grams of nabr will react with 311 grams of pb(no3)2?
pb(no3)2(aq)+2nabr(g) -> pbbr2(s) + 2nano3(aq)
the molar mass of nabr is 102.9 grams and that of pb(no3)2 is 331.21 grams.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
In the following reaction, how many grams of nabr will react with 311 grams of pb(no3)2?
pb(no...
pb(no...
Questions

English, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00

Mathematics, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00


History, 18.11.2020 22:00

Mathematics, 18.11.2020 22:00


English, 18.11.2020 22:00

Mathematics, 18.11.2020 22:00


Mathematics, 18.11.2020 22:00


English, 18.11.2020 22:00

Mathematics, 18.11.2020 22:00



 of particles.
of particles.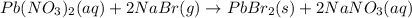
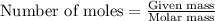

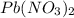 reacts with 2 moles of
reacts with 2 moles of 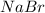
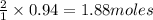 of
of