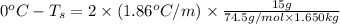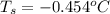Chemistry, 30.07.2019 02:00 melinda12ms
Calculate the freezing point of a solution containing 15 grams of kcl and 1650.0 grams of water. the molal-freezing-point-depression constant (kf) for water is 1.86 ∘c/m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
Calculate the freezing point of a solution containing 15 grams of kcl and 1650.0 grams of water. the...
Questions

Mathematics, 17.02.2022 08:20





English, 17.02.2022 08:20

Chemistry, 17.02.2022 08:20

Mathematics, 17.02.2022 08:20

English, 17.02.2022 08:20

English, 17.02.2022 08:20

English, 17.02.2022 08:20


Mathematics, 17.02.2022 08:20

Geography, 17.02.2022 08:20



English, 17.02.2022 08:20



Mathematics, 17.02.2022 08:20

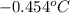
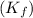 for water =
for water = 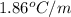
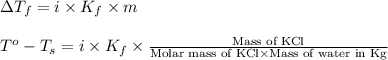
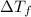 = change in freezing point
= change in freezing point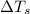 = freezing point of solution = ?
= freezing point of solution = ?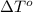 = freezing point of water =
= freezing point of water = 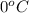
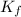 = freezing point constant for water =
= freezing point constant for water =