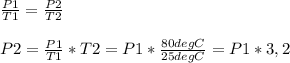Chemistry, 29.07.2019 22:30 davidgarcia522083
Asample of gas in a sealed container (fixed volume) is heated from room temperature to 80.0°c. a. does the pressure inside the container increase or decrease? b. the effect of temperature on the pressure of a gas illustrates 's law. c. explain what happens at the molecular level to change the pressure as the temperature is raised.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Asample of gas in a sealed container (fixed volume) is heated from room temperature to 80.0°c. a. do...
Questions

History, 25.08.2019 05:30





Computers and Technology, 25.08.2019 05:30


Mathematics, 25.08.2019 05:30


Chemistry, 25.08.2019 05:30

Mathematics, 25.08.2019 05:30




History, 25.08.2019 05:30

Mathematics, 25.08.2019 05:30

Biology, 25.08.2019 05:30

English, 25.08.2019 05:30