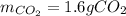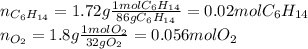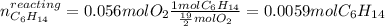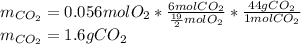Problem page liquid hexane ch3ch24ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.72 g of hexane is mixed with 1.8 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1


Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Problem page liquid hexane ch3ch24ch3 will react with gaseous oxygen o2 to produce gaseous carbon di...
Questions

Mathematics, 03.02.2021 01:50

Mathematics, 03.02.2021 01:50

Mathematics, 03.02.2021 01:50

Mathematics, 03.02.2021 01:50


Mathematics, 03.02.2021 01:50

Mathematics, 03.02.2021 01:50


Mathematics, 03.02.2021 01:50

English, 03.02.2021 01:50


Mathematics, 03.02.2021 01:50

Chemistry, 03.02.2021 01:50




English, 03.02.2021 01:50



Mathematics, 03.02.2021 01:50