
Chemistry, 29.07.2019 16:30 damientran
Can someone . a sucrose solution is prepared to a final concentration of 0.170m . convert this value into terms of g/l, molality, and mass % (molecular weight, mwsucrose = 342.296g/mol ; density, ρsol′n = 1.02g/ml ; mass of water, mwat = 961.8g ). note that the mass of solute is included in the density of the solution,

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Can someone . a sucrose solution is prepared to a final concentration of 0.170m . convert this valu...
Questions




Mathematics, 03.04.2020 18:49




Arts, 03.04.2020 18:49

Mathematics, 03.04.2020 18:50




English, 03.04.2020 18:50







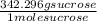 = 58.2 g/L
= 58.2 g/L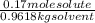 = 0.177 mole / kg
= 0.177 mole / kg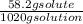 x 100 = 5.7 %
x 100 = 5.7 %

