
Chemistry, 29.07.2019 16:30 julieariscar769
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar from your average value for the mass percentage of acetic acid in vinegar. density of vinegar= 1.005 g/ml average mass % acetic acid= 5.2%,

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar...
Questions













Mathematics, 27.06.2020 01:01


Mathematics, 27.06.2020 01:01

History, 27.06.2020 01:01




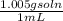 =1005 g soln
=1005 g soln

