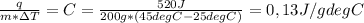Chemistry, 29.07.2019 14:00 1tallison1
Will mark as brainliest. two hundred grams of a substance requires 0.52 kj of heat to raise its temperature from 25°c to 45°c. use the table to identify the substance. q = mc▲t. mass (m) is in grams. temperature is in degrees celsius. substance: specific heat (c) in joules per gram/degrees centigrade: water (ice) 2.05 iron 0.46 aluminium 0.90 gold 0.13 copper 0.39 ammonia (liquid) 4.70 ethanol 2.44 gasoline 2.22 water (liquid) 4.18 water (vapor) 2.08 air (25 degrees celsius) 1.01 oxygen 0.92 hydrogen 14.30 question options: water gasoline ammonia gold

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Will mark as brainliest. two hundred grams of a substance requires 0.52 kj of heat to raise its temp...
Questions




Mathematics, 23.11.2019 11:31



Mathematics, 23.11.2019 11:31

Physics, 23.11.2019 11:31


English, 23.11.2019 11:31

Biology, 23.11.2019 11:31




Mathematics, 23.11.2019 11:31

History, 23.11.2019 11:31

Health, 23.11.2019 11:31



Biology, 23.11.2019 11:31