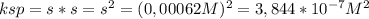(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the dissociation of agcl(s) in water. (ii) calculate the solubility, in mol l-1, of agcl(s) in water at 10°c. (iii) calculate the value of the solubility-product constant, ksp, for agcl(s) at 10°c. (b) at 25°c, the value of ksp for pbcl2(s) is 1

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the...
Questions










Computers and Technology, 14.12.2019 06:31

Computers and Technology, 14.12.2019 06:31









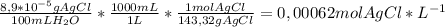
![ksp= [Ag^{+}]*[Cl^{-}]](/tpl/images/0144/2915/6d867.png)