
Chemistry, 28.07.2019 21:30 damilysgonzalez2
For the balanced equation shown below, how many moles of o2 will react with 0.3020 moles of co2? 2c2h5oh + 6o2 → 4co2 + 6h2o question 9 options:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
For the balanced equation shown below, how many moles of o2 will react with 0.3020 moles of co2? 2c...
Questions



History, 28.12.2021 18:40

Mathematics, 28.12.2021 18:40



SAT, 28.12.2021 18:40

English, 28.12.2021 18:40



Physics, 28.12.2021 18:50


SAT, 28.12.2021 18:50

SAT, 28.12.2021 18:50


SAT, 28.12.2021 18:50

SAT, 28.12.2021 18:50



Chemistry, 28.12.2021 18:50

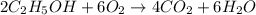
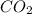 are produced from= 6 moles of
are produced from= 6 moles of 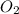
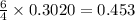 moles of
moles of 

