These equations below show two standard reduction potentials.
which value represents the...

Chemistry, 12.01.2020 05:31 coralstoner6793
These equations below show two standard reduction potentials.
which value represents the standard cell potential of the spontaneous overall reaction?
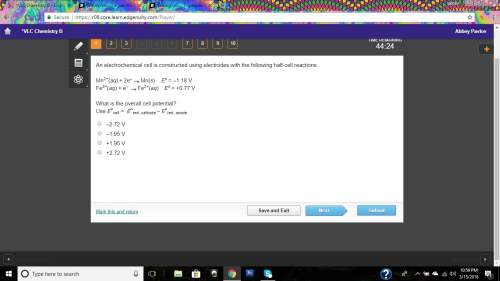

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

You know the right answer?
Questions

Physics, 21.11.2020 20:20

Social Studies, 21.11.2020 20:20

Physics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

History, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20



Mathematics, 21.11.2020 20:20

Mathematics, 21.11.2020 20:20

English, 21.11.2020 20:20



Mathematics, 21.11.2020 20:30

Mathematics, 21.11.2020 20:30



