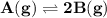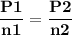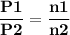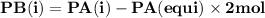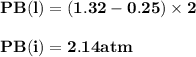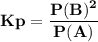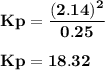Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


You know the right answer?
For the reaction a(g) 2 b(g), a reaction vessel initially contains only a at a pressure of pa = 1.32...
Questions

Mathematics, 03.06.2020 23:58

Spanish, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58


Chemistry, 03.06.2020 23:58


History, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58

History, 03.06.2020 23:58

Arts, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58


English, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58


English, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58

Mathematics, 03.06.2020 23:58