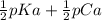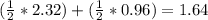Chemistry, 28.07.2019 07:00 hewonabi123
Saccharin, a sugar substitute, is a weak acid with pka=2.32 at 25 ∘c. it ionizes in aqueous solution as follows: hnc7h4so3(aq)←−→h+(aq)+nc7h4so−3(aq ) part a what is the ph of a 0.11 m solution of this substance?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
Saccharin, a sugar substitute, is a weak acid with pka=2.32 at 25 ∘c. it ionizes in aqueous solution...
Questions

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01


English, 16.10.2020 22:01


Biology, 16.10.2020 22:01


Business, 16.10.2020 22:01

Business, 16.10.2020 22:01

Social Studies, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01


Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01





Advanced Placement (AP), 16.10.2020 22:01