
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water and results in a decrease in the water’s temperature. which of the following best describes the difference in the potential energy of the reactants and products in this reaction? the potential energy remains low before and after the reaction. the potential energy of the reactants is higher than that of the products. the potential energy of the reactants is much lower than that of the products the potential energy of the reactants does not differ from that of the products.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water a...
Questions




Mathematics, 28.01.2020 18:56



Physics, 28.01.2020 18:56


Mathematics, 28.01.2020 18:56

Mathematics, 28.01.2020 18:56


Mathematics, 28.01.2020 18:56






Mathematics, 28.01.2020 18:56


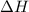 for the reaction comes out to be positive.
for the reaction comes out to be positive.


