Chemistry, 28.07.2019 01:30 mallyosburn
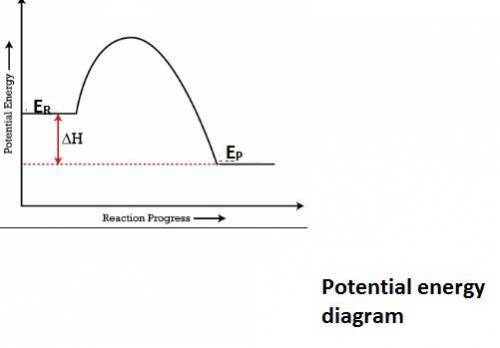
In an exothermic reaction, the potential energy of the products is lower than that of the reactants. which of the following is also true for an exothermic reaction? the enthalpy change is negative it requires a catalyst to take place energy is absorbed from the surroundings the kinetic energy of the products is also lower

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
In an exothermic reaction, the potential energy of the products is lower than that of the reactants....
Questions

Mathematics, 16.07.2021 21:50

Engineering, 16.07.2021 21:50

Biology, 16.07.2021 21:50

Mathematics, 16.07.2021 21:50



Spanish, 16.07.2021 21:50








Computers and Technology, 16.07.2021 21:50




Social Studies, 16.07.2021 21:50

Mathematics, 16.07.2021 21:50

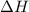 for the reaction comes out to be positive.
for the reaction comes out to be positive.