Chemistry, 27.07.2019 17:30 nockturnal1993
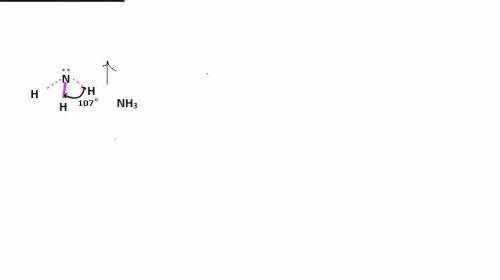
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride has a central boron atom bonded to three fluorine atoms. however, ammonia is pyramidal and boron trifluoride is trigonal planar in shape. which statement justifies this difference in their structure? the lone pair of electrons in boron trifluoride increases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride decreases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride stabilizes the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in ammonia increases the bond angle between the bonding pairs, giving it a pyramidal structure. the lone pair of electrons on the nitrogen in ammonia decreases the bond angle between the bonding pairs, giving it a pyramidal structure.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride...
Questions






Biology, 19.02.2020 23:16






Biology, 19.02.2020 23:17

Social Studies, 19.02.2020 23:17


Social Studies, 19.02.2020 23:17



Social Studies, 19.02.2020 23:17

Biology, 19.02.2020 23:17

![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0139/5742/08b6a.png)
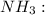
![{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0139/5742/46c6e.png)
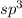 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
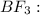
![{\text{Number of electrons}} =\frac{1}{2}[3+3-0+0]=3](/tpl/images/0139/5742/beb45.png)
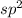 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.