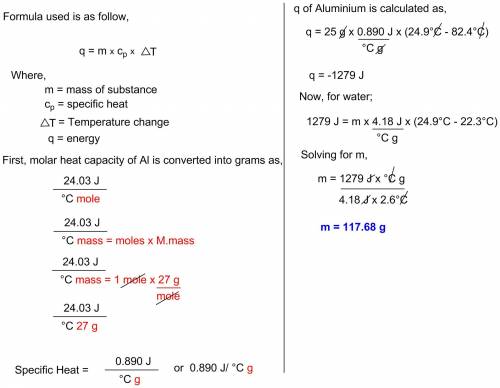
A12.8 g piece of aluminum (which has a molar heat capacity of 24.03 j/°c·mol) is heated to 82.4°c and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/g°c) initially at 22.3°c. the final temperature of the water is 25.7°c. ignoring significant figures, calculate the mass of water in the calorimeter.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
A12.8 g piece of aluminum (which has a molar heat capacity of 24.03 j/°c·mol) is heated to 82.4°c an...
Questions

Spanish, 05.11.2020 22:10



Computers and Technology, 05.11.2020 22:10

History, 05.11.2020 22:10

Chemistry, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10




English, 05.11.2020 22:10

Biology, 05.11.2020 22:10



Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10



Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10