
Chemistry, 16.01.2020 05:31 skylerdemi1
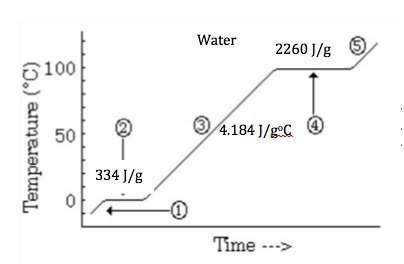
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusion.
a) the potential energy of fusion represents a greater change of stored energy.
b) liquid water is stabilized by strong intermolecular forces between the molecules.
c) the kinetic energy of the water molecules is so much greater in the liquid state.
d) the bonding forces for solid water are much stronger than the bonding in the liquid.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
Elaborate the reason that the heat of vaporization of water is so much greater than its heat of fusi...
Questions

Health, 25.06.2019 11:30

Geography, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30

Chemistry, 25.06.2019 11:30

English, 25.06.2019 11:30



Advanced Placement (AP), 25.06.2019 11:30

Physics, 25.06.2019 11:30

Mathematics, 25.06.2019 11:30



Mathematics, 25.06.2019 11:30


Mathematics, 25.06.2019 11:30

Mathematics, 25.06.2019 11:30



Mathematics, 25.06.2019 11:30



