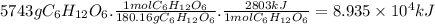Chemistry, 27.07.2019 13:00 rakanmadi87
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +2803 kj/mol calculate the solar energy required to produce 5743 g of c6h12o6.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Given the thermochemical equation for photosynthesis, 6h2o(l) + 6co2(g) → c6h12o6(s) + 6o2(g) δh = +...
Questions

Mathematics, 09.07.2021 01:00


Mathematics, 09.07.2021 01:00

Mathematics, 09.07.2021 01:00






Mathematics, 09.07.2021 01:00

Mathematics, 09.07.2021 01:00





Mathematics, 09.07.2021 01:00

Mathematics, 09.07.2021 01:00