
Chemistry, 27.07.2019 13:00 Clervoyantyvonne
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) according to one acid-base theory, the forward reaction is classified as an acid-base reaction because

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

You know the right answer?
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) ac...
Questions

Mathematics, 01.03.2021 03:20


Business, 01.03.2021 03:20

Physics, 01.03.2021 03:20



Chemistry, 01.03.2021 03:20

Mathematics, 01.03.2021 03:20

Biology, 01.03.2021 03:20


Mathematics, 01.03.2021 03:30


Mathematics, 01.03.2021 03:30



Mathematics, 01.03.2021 03:30


Mathematics, 01.03.2021 03:30

English, 01.03.2021 03:30

History, 01.03.2021 03:30

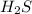 loses proton and
loses proton and 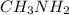 accepts proton
accepts proton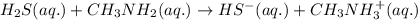
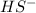 which is a conjugate base.
which is a conjugate base.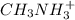 which is a conjugate acid.
which is a conjugate acid.

