
Chemistry, 27.07.2019 12:00 Nicoleebel2984
Acalorimeter contains 100 g of water at 39.8 ºc. a 8.23 g object at 50 ºc is placed inside the calorimeter. when equilibrium has been reached, the new temperature of the water and metal object is 40 ºc. what type of metal is the object made from?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

You know the right answer?
Acalorimeter contains 100 g of water at 39.8 ºc. a 8.23 g object at 50 ºc is placed inside the calor...
Questions






Mathematics, 28.06.2020 05:01






Computers and Technology, 28.06.2020 05:01








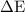 water =
water = 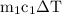 =
= 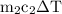
 4.814
4.814 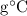 .
.

