Chemistry, 27.07.2019 08:30 xxxamslashxxx9
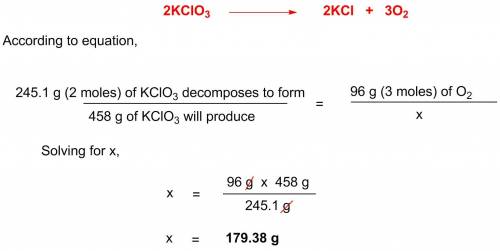
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2, if you start with 458 g of kclo3, what mass (g) of o2 will be produced? a. 83.71 l o2 b. 179.39 l o2 c. 687 l o2 d. 67.2 l o2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Potassium chlorate decomposes according to the following chemical equation: 2kclo3 > 2kcl + 3o2...
Questions

Mathematics, 18.08.2019 19:00


Mathematics, 18.08.2019 19:00

History, 18.08.2019 19:00

Health, 18.08.2019 19:00




History, 18.08.2019 19:00


Social Studies, 18.08.2019 19:00


English, 18.08.2019 19:00

Mathematics, 18.08.2019 19:00


Mathematics, 18.08.2019 19:00


Mathematics, 18.08.2019 19:00


History, 18.08.2019 19:00