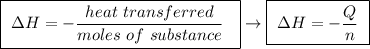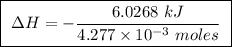Chemistry, 27.07.2019 07:00 alejandra216
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.44 k. calculate the enthalpy change per mole of ethylene combusted.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.120-g sample of ethylene (c2h4) was bur...
Questions

Mathematics, 04.02.2020 02:44




Mathematics, 04.02.2020 02:44


Mathematics, 04.02.2020 02:44

Mathematics, 04.02.2020 02:44




Mathematics, 04.02.2020 02:44

Mathematics, 04.02.2020 02:44


Mathematics, 04.02.2020 02:44

Mathematics, 04.02.2020 02:44

Mathematics, 04.02.2020 02:44


Mathematics, 04.02.2020 02:44

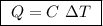
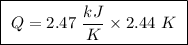
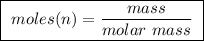
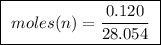
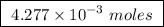 of ethylene.
of ethylene.