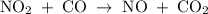The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no2 + no2 no + no3 step 2: no3 + co no2 + co2 the experimental rate law is rate = k[no2]2. (a) write the equation for the overall reaction. do not include phase abbreviations. (b) identify the intermediate(s). no3 no2 co no co2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no...
Questions


Biology, 24.10.2021 02:10





Mathematics, 24.10.2021 02:10

History, 24.10.2021 02:10


SAT, 24.10.2021 02:10


Mathematics, 24.10.2021 02:10

Mathematics, 24.10.2021 02:10

Biology, 24.10.2021 02:10

Mathematics, 24.10.2021 02:10


English, 24.10.2021 02:10

Computers and Technology, 24.10.2021 02:10


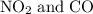 to produce NO and
to produce NO and 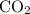 occurs in the given two steps.
occurs in the given two steps.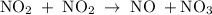

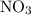 because it act as an intermediate and is utilized in the next step.
because it act as an intermediate and is utilized in the next step.