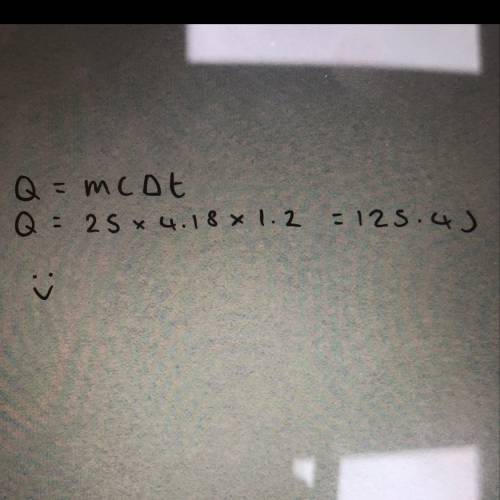Asmall pebble is heated and placed in a foam cup calorimeter containing 25.0 ml of water at 23.0°c. the water reaches a maximum temperature of 24.2°c. how many joules of heat were released by the pebble? (the specific heat capacity of water is 4.18 j/g•°c and the density of water is 1.00 g/ml)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Asmall pebble is heated and placed in a foam cup calorimeter containing 25.0 ml of water at 23.0°c....
Questions


English, 30.03.2021 21:30





Mathematics, 30.03.2021 21:40


History, 30.03.2021 21:40


Mathematics, 30.03.2021 21:40

Mathematics, 30.03.2021 21:40

Physics, 30.03.2021 21:40

Mathematics, 30.03.2021 21:40



Mathematics, 30.03.2021 21:40

Physics, 30.03.2021 21:40