

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Calculate the ph of 100 ml of a buffer solution containing 0.05 m benzoic acid (c6h5co2h; ka = 6.4...
Questions





Mathematics, 09.04.2020 19:22

Mathematics, 09.04.2020 19:22

Mathematics, 09.04.2020 19:22

History, 09.04.2020 19:22



History, 09.04.2020 19:22



Mathematics, 09.04.2020 19:22


Mathematics, 09.04.2020 19:22

History, 09.04.2020 19:22



History, 09.04.2020 19:22

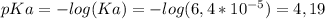 )
)![pH=pKa + log ( \frac{[A^{-}] }{[HA]} ) \\ \\ pH=4,19 + log ( \frac{0,05M}{0,05M}) \\ \\ pH=4,19](/tpl/images/0137/1803/3e5ee.png)


