
Chemistry, 27.07.2019 01:00 mamieengler
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
The equation below shows hydrogen reacting with oxygen to produce water. 2h2+o2> 2h2o if 16mol of...
Questions

English, 04.01.2021 18:00


Mathematics, 04.01.2021 18:00


Mathematics, 04.01.2021 18:00

Mathematics, 04.01.2021 18:00


Mathematics, 04.01.2021 18:00

Geography, 04.01.2021 18:00

English, 04.01.2021 18:00




Mathematics, 04.01.2021 18:10

Arts, 04.01.2021 18:10




Mathematics, 04.01.2021 18:10

Mathematics, 04.01.2021 18:10

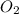 = 16 mole
= 16 mole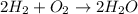
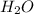
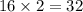 moles of
moles of 

