
Chemistry, 26.07.2019 20:30 wyattgrubb00
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca? a) (1 mol ca/6.02 x 1023 atoms ca)(40.08 g ca/1 mol ca) b) (1 mol ca/4.23 x 1012 atoms ca)(40.08 g ca/1 mol ca) c) (4.23 x 1012 atoms ca/1 mol ca)(1 mol ca/40.08 g ca) d) 4.23 x 1012 atoms ca/6.02 x 1023 atoms ca)(40.08 g ca)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca?...
Questions

History, 22.06.2019 16:00

History, 22.06.2019 16:00




History, 22.06.2019 16:00


History, 22.06.2019 16:00

History, 22.06.2019 16:00



History, 22.06.2019 16:00

History, 22.06.2019 16:00

Biology, 22.06.2019 16:00


Mathematics, 22.06.2019 16:00



Mathematics, 22.06.2019 16:00

Health, 22.06.2019 16:00

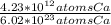 * 40.08 g Ca
* 40.08 g Ca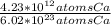 * 40.08 g Ca
* 40.08 g Ca

