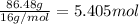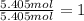Chemistry, 26.07.2019 18:30 ashley54899
200 grams of an organic sample which contains only carbon, hydrogen, and oxygen is analyzed and found to contain 97.30 grams of carbon, 16.22 grams of hydrogen and the remainder oxygen. what is the empirical formula for the compound?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
200 grams of an organic sample which contains only carbon, hydrogen, and oxygen is analyzed and foun...
Questions


Computers and Technology, 28.10.2020 22:10

History, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10


Mathematics, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10

English, 28.10.2020 22:10



Mathematics, 28.10.2020 22:10

English, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10


Mathematics, 28.10.2020 22:10

History, 28.10.2020 22:10

Mathematics, 28.10.2020 22:10

English, 28.10.2020 22:10