
Chemistry, 26.07.2019 18:00 yhbgvfcd331
The specific heat of water is 4.18 j/(g⋅∘c). calculate the molar heat capacity of water. express your answer to three significant figures and include the appropriate units.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
The specific heat of water is 4.18 j/(g⋅∘c). calculate the molar heat capacity of water. express you...
Questions

Health, 19.09.2019 09:10




Business, 19.09.2019 09:20


Mathematics, 19.09.2019 09:20

Mathematics, 19.09.2019 09:20

Mathematics, 19.09.2019 09:20


History, 19.09.2019 09:20

Mathematics, 19.09.2019 09:20

History, 19.09.2019 09:20

English, 19.09.2019 09:20



Mathematics, 19.09.2019 09:20


Mathematics, 19.09.2019 09:20

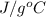 . Now, we will calculate the molar mass water as follows.
. Now, we will calculate the molar mass water as follows.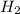 =
= 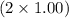 g/mol = 2.00 g.mol
g/mol = 2.00 g.mol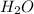 = (15.99 + 2.00) g/mol
= (15.99 + 2.00) g/mol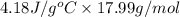
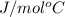
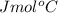 .
.


