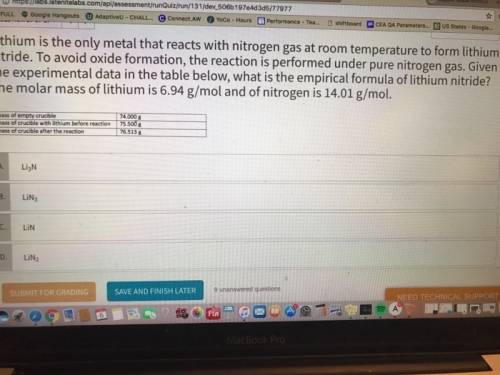
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride. to avoid oxide formation, the reaction is performed under pure nitrogen gas. given the experimental data in the table below, what is the empirical formula of lithium nitride? the molar mass of lithium is 6.94 g/mol and of nitrogen is 14.01 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride....
Questions




World Languages, 17.06.2020 22:57




Mathematics, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57


Mathematics, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57


Mathematics, 17.06.2020 22:57


History, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57


Mathematics, 17.06.2020 22:57

Physics, 17.06.2020 22:57