
Chemistry, 26.07.2019 15:30 DOGENINJA4542
Morphine has the formula c17h19no3. it is a base and accepts one proton per molecule. it is isolated from opium. a 0.685 −g sample of opium is found to require 8.91 ml of a 1.16×10−2 m solution of sulfuric acid for neutralization. part a assuming that morphine is the only acid or base present in opium, calculate the percent morphine in the sample of opium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Morphine has the formula c17h19no3. it is a base and accepts one proton per molecule. it is isolated...
Questions

History, 14.04.2020 18:56






Mathematics, 14.04.2020 18:56

Mathematics, 14.04.2020 18:56



History, 14.04.2020 18:56


Arts, 14.04.2020 18:56

Social Studies, 14.04.2020 18:56

English, 14.04.2020 18:56





Mathematics, 14.04.2020 18:56

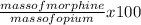
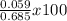 = 8.6 %
= 8.6 % 

